Background : Patients with myelodysplastic syndromes (MDS) can experience fatigue, infection, anemia, bruising, and bleeding. Fatigue and physical functioning (PF) are often the most adversely impacted areas of patient health-related quality of life. The objective of this study was to generate quantitative evidence to determine whether the PF score from the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) and the Fatigue score from the Patient-Reported Outcome Measurement Information System - Fatigue Short Form 7a (PROMIS SF 7a) measures are suitable candidates for developing patient-reported endpoints for clinical trials in treatment-naïve patients with higher-risk MDS (HR-MDS). We also aimed to derive definitions for meaningful change for both PF and Fatigue scores to aid in interpretation of efficacy analyses using these scores.
Methods : This analysis used aggregate data from the Phase 3 M15-954 study (NCT04401748), which is an ongoing, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of venetoclax in combination with azacitidine in treatment-naïve adult patients with HR-MDS. The EORTC QLQ-C30 and the PROMIS SF 7a questionnaires were administered on the first day of each 28-day cycle for the first 7 cycles and every 3 cycles thereafter, and at the treatment-completion visit. For both measures, the analyses evaluated item characteristics at baseline, whether the hypothesized structure was consistent with the scoring algorithms implemented in the trial, and the reliability and validity of scores; subsequently, definitions for meaningful improvement or deterioration were determined. Anchor-based analyses were used to generate definitions for meaningful deterioration or improvement from baseline to Cycle 4 Day 1 for EORTC QLQ-C30 PF domain, and Cycle 7 Day 1 for PROMIS SF 7a. Construct-aligned anchors were used for these analyses, with a change in Patient Global Impression of Severity (PGIS) - Physical Activities and in Patient Global Impression of Change (PGIC) - Physical Activities used as the anchor for the analyses of the EORTC QLQ-C30 PF scores and PGIS-Fatigue and PGIC-Fatigue used for the analyses of the PROMIS SF 7a scores.
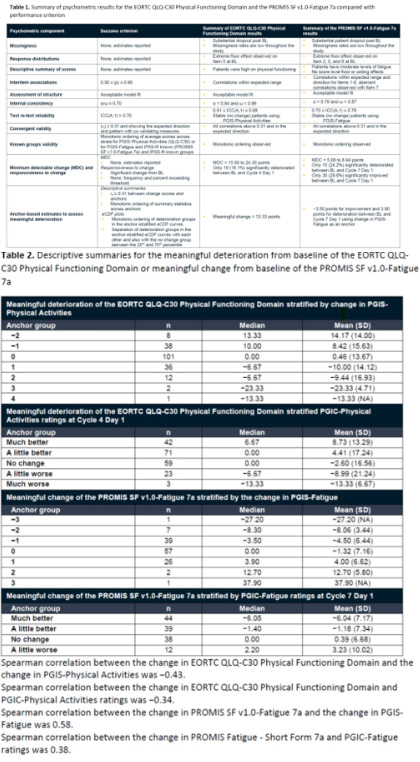
Results : Approximately 500 treatment-naïve higher-risk patients with MDS enrolled in the study were included in this analysis. Both EORTC QLQ-C30 PF and PROMIS SF 7a measures demonstrated adequate reliability and validity; both measures exceeded the cut-off for acceptable internal consistency reliability, test re-test reliability, and convergent validity with co-validators ( Table 1). The definition for deterioration for the EORTC QLQ-C30 PF domain was −13.33 (rounded to the nearest possible incremental change score), which was corroborated with the PGIC-Physical Activities ( Table 2). The correlation between the EORTC QLQ-C30 PF domain and PGIS-Physical Activities scores was −0.43, and the correlation between the EORTC QLQ-C30 PF domain and PGIC-Physical Activities scores was −0.34, indicating that these measures were sufficient to be used as anchors. For PROMIS SF 7a, the definition for deterioration and improvement was 3.90 and −3.50, respectively. Both results were also corroborated with the PGIC-Fatigue. The correlation between the PROMIS SF 7a and PGIS-Fatigue scores was 0.58, and the correlation between the PROMIS SF 7a and PGIC-Fatigue scores was 0.38, indicating that these measures were sufficient to be used as anchors.
Conclusions : The psychometric evaluation of EORTC QLQ-C30 PF and PROMIS SF 7a Fatigue scores from M15-954 study's blinded data provides sufficient evidence of test re-test reliability, construct validity, responsiveness, and score interpretability and supports their use as endpoints for clinical trials in treatment-naïve HR-MDS patients for regulatory decision-making.
Disclosures
Lyons:Incyte Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Exact Sciences: Research Funding; Pfizer: Research Funding; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas Pharma: Research Funding; Texas Oncology: Current holder of stock options in a privately-held company; McKesson: Other: Leadership; Lessen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Foster:Lumanity: Current Employment. Jewett:Lumanity: Current Employment. Liu:Lumanity: Current Employment. Sen:AbbVie Inc: Current Employment, Current holder of stock options in a privately-held company. Bui:AbbVie Inc: Current Employment, Current holder of stock options in a privately-held company. Kamalakar:AbbVie Inc: Current Employment, Current holder of stock options in a privately-held company. Potluri:AbbVie: Current Employment, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal